Common respiratory virus manipulates immune genes to protect itself
Findings could lead to better therapies for respiratory syncytial virus infection
 Leung and Brody Labs
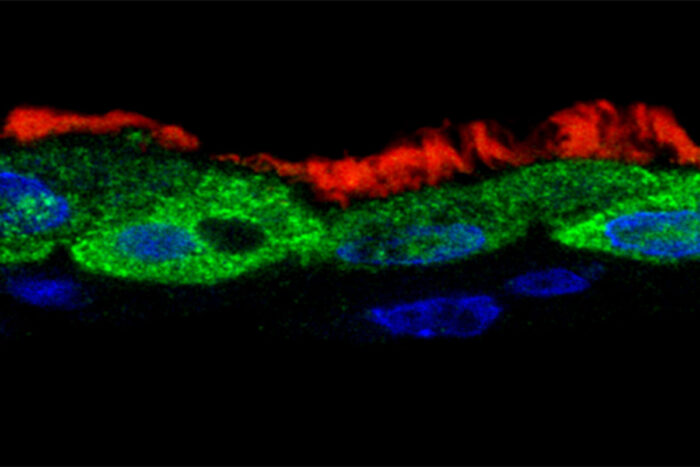
Leung and Brody LabsThe viral protein NS1 (green) is found inside the nuclei (blue) of ciliated human respiratory tract cells (cilia shown in red) that have been infected with respiratory syncytial virus (RSV). Researchers at Washington University School of Medicine in St. Louis have discovered that NS1 alters the activity of immune genes, sabotaging the immune response to RSV infection.
Nearly everyone gets infected with respiratory syncytial virus (RSV) repeatedly over the course of a lifetime, starting in childhood. Most times, people fight off the virus handily and only end up with a mild cold. But some people — most often young children experiencing their first infection or older adults whose immunity has waned — develop pneumonia or bronchiolitis, serious lung infections that can lead to hospitalization and sometimes death.
Researchers at Washington University School of Medicine in St. Louis have figured out how the virus undermines the body’s defenses, a step toward understanding why the virus is capable of causing serious illness in vulnerable populations. They discovered that the virus produces a protein — called nonstructural protein 1, or NS1 — that slips inside the nucleus and alters the activity of immune genes, sabotaging the immune response.
The findings, published Oct. 12 in Cell Reports, point toward new strategies to prevent or treat RSV infection, and may even provide clues to why severe cases of RSV put people at elevated risk of developing asthma.
“RSV is a significant health burden. It leads to thousands of hospitalizations and a significant number of deaths in the U.S. every year, and there aren’t many effective therapies or any vaccines currently available for it,” said co-senior author Daisy W. Leung, PhD, an associate professor of medicine, of biochemistry & molecular biophysics, and of pathology & immunology. “NS1 is an important part of the reason RSV is capable of causing disease. Not only does the protein interfere with the immune response, it is also important for viral replication. I think the work that we describe in this paper provides a basis for targeting NS1 therapeutically or for vaccine development.”
RSV is a very common virus. Every year in the U.S., about 58,000 children under age 5 are hospitalized due to RSV infection, and 100 to 500 infected children die. Children who survive a serious case of RSV are 30% to 40% more likely than the general population to develop recurrent wheezing or asthma. The virus also kills about 14,000 older adults every year.
Before this study, RSV researchers already had NS1 on their radars as one of the weapons used by the virus to counter the body’s defenses. In 2017, Leung published a paper in Nature Microbiology identifying the precise part of the protein involved in undermining the immune response. But it wasn’t clear how the protein was doing so.
To find out, co-first author Jingjing Pei, PhD, then a postdoctoral researcher in Leung’s lab, infected cells taken from a person’s respiratory tract with RSV. Then, she used an antibody against NS1 that the Leung lab and collaborators on the study developed to track where the protein went inside the cells. She found that while the virus genome and other viral proteins stayed in the main part of the cell and produced more copies of the virus, NS1 sneaked into the nucleus.
Further experiments by co-first author Nina R. Beri, PhD, revealed what NS1 was doing in the nucleus: sabotaging the cell’s antiviral efforts by altering the expression of its immune genes. Beri, who has since graduated, conducted the experiments as a graduate student in the lab of co-senior author Jacqueline E. Payton, MD, PhD, an assistant professor of pathology & immunology.
“NS1 wasn’t just floating around the nucleus, it was interacting with the proteins that regulate gene expression,” Payton said. “The group of genes most affected were the immune-response genes whose expression gets turned on really high when a cell is infected by a virus. It was binding right at the spots on the genome that control expression – the same ones that you’d expect if it were trying to interfere with the immune response.”
By illuminating the details of how NS1 manipulates gene expression, this study provides crucial data that could aid efforts to target the protein for drug or vaccine development. It may even provide a clue to the link between RSV and asthma. The key, Payton suggested, may lie in the epigenome, the pattern of chemical units attached to DNA that influence gene expression.
“Once a cell — any cell, not just an immune cell — encounters an infection, its epigenome changes and primes it to be able to respond more quickly the next time it encounters an infection,” Payton said. “My theory is that NS1 may alter the epigenome in susceptible patients such that the next time they encounter RSV — or maybe even just dust or cat dander — they have an aberrant inflammatory response that is damaging rather than protective. That is an idea we are exploring now.”